by Claire Robinson
A Monsanto genetically modified (GMO) maize, called MON89034, caused kidney disease and bladder stones in rats in industry tests performed in 2007. Several EU member states, including Germany, Belgium, Austria, and France, independently raised concerns about these results during the EU’s standard three-month regulatory consultation process. But the central GMO regulator of the EU, the European Food Safety Authority (EFSA), issued a favourable opinion on MON89034 regardless.[1] With the usual lack of agreement from EU member states on whether to authorize the maize, the EU Commission subsequently approved MON89034 for human consumption in 2011.[2]
MON89034 has since been crossed with other GM maize varieties to form “stacked” GM crops containing multiple GM traits. As each newly stacked GMO trait involving MON89034 has come up for approval, member states have continued to draw attention to the original adverse health impacts in the rats fed MON89034.
France in particular has repeatedly raised concerns about the kidney and bladder effects in rats fed MON89034. But EFSA has always disregarded them and issued favourable opinions, allowing the EU leadership (the Commission) to so far approve nine stacked maize varieties containing MON89034.[3]
What’s in MON89034 maize?
MON89034, marketed as YieldGard™ VT Pro™, is a Monsanto GM maize that contains its own Bt toxin insecticides (also known as Cry toxins). MON89034 maize plants produce a synthetic Bt toxin, Cry1A.105 – a combination of Bt toxins called Cry1Ac/Cry1Ab and Cry1F. This synthetic toxin is combined with another Bt toxin called Cry2Ab2 [4]. Neither protein has a natural equivalent, so safety cannot be concluded by comparison with Bt toxins used previously in agriculture (Latham et al., 2017).
Kidney and bladder adverse effects
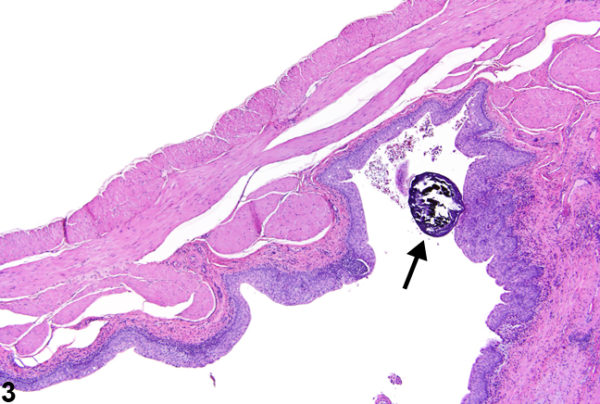
EFSA asserted that this GM maize was as safe as non-GMO maize even though in Monsanto’s own studies two out of 20 female rats receiving the high dose of GM maize (33% of the total diet) developed kidney damage and bladder stones. One of the two rats died after just two weeks on the GM diet.
EFSA reported that the changes in the two rats included “minimal” chronic progressive kidney disease or damage, minimal to moderate transitional cell hyperplasia (cell proliferation that can be a precursor to cancer of the urinary system), inflammation, and hydronephrosis (presence of water in the kidneys due to obstruction). EFSA added that both rats had “urinary bladder calculi” – bladder stones. The study pathologist concluded that the tissue abnormalities observed were probably linked to the stones.
EFSA’s response
Nevertheless, EFSA concluded that the kidney damage and bladder stones in the GM maize-fed rats were “spontaneous” and had nothing to do with the GM diet. This was because, according to EFSA, “a low incidence of urinary bladder calculi [stones] is known to occur in this rat strain”.
EFSA added, “According to historical control data supplied in the application, the incidence of urinary bladder calculi in high dose females in this study was also found in female control rats in previous studies conducted with CD rats in the same testing laboratory. The [GMO] Panel therefore considers the urinary bladder calculi as well as the associated kidney alterations as incidental findings which were not related to administration of maize MON89034.”
Member state concerns
EFSA issued its favourable opinion on MON89034 in 2008, concluding that it was as safe as non-GMO maize.[1] Some EU member states strongly disagreed. This is clear from their comments from the three month consultation period leading up to the publication of EFSA’s opinion.[5]
Germany commented on the kidney and bladder results: “While the applicant suggests that these findings are unrelated to consumption of the test diet, possible adverse effects of MON89034 maize on the health of the test animals cannot be excluded from this result and from other significantly different nephrological [kidney] and haematological [blood] findings in MON89034 maize fed female rats.”[5]
The German experts, from the German Federal Agency for Nature Conservation (BfN), recommended that Monsanto be required to carry out an additional two-generation study with the maize to test for long-term health effects. But EFSA responded that it saw “no reason to request additional feeding studies”.[5]
Austria also voiced concerns about the kidney and bladder findings, as did the experts from Belgium’s Biosafety Advisory Council. The Belgian experts focused on an additional worrying finding: a low thyroid/parathyroid weight relative to final body weight was found in this same group of female rats fed a 33% GM maize diet. Referring to the whole roster of effects in the GM-fed rats, Belgium recommended having “a closer look” to see “whether these findings are solely due to chance. In case any doubt remains, further testing is recommended.”[5]
Historical control data
Much of the disagreement around MON89034 surrounded the interpretation and reliability of data from historical controls. Thus EFSA repeatedly dismissed member states’ concerns about the bladder stones in the GM maize-fed female rats by referring to historical data that Monsanto had submitted from control group rats in other experiments. These data are unpublished and so cannot be checked by independent scientists. EFSA claimed that the data show that in this strain of rat, bladder stones or urinary calculi “sometimes appear”.[5] The implication was that MON89034 did not cause the problems.
However, the experts from the French food safety agency ANSES pointed out that the percentage of high-dose GM maize-fed rats developing bladder stones was 10%, whereas it was 0.49% in the historical control data.[5] EFSA never addressed this major discrepancy.
The French experts commented that “additional explanations” were required. Without them, ANSES “cannot conclude on the safety of products derived from maize varieties carrying the MON89034 transformation event”.[5]
The French were still not reassured by Monsanto’s historical control data in 2016,[6] when they reviewed Monsanto’s application for the approval of a GM maize containing the MON89034 trait, called MIR162 x MON87427 x MON89034 x NK603.
ANSES stated: “Although historical data from 70 studies conducted between 1999 and 2006 with rats of the same strain were provided by the petitioner, they did not appear sufficient to conclude that there was no link between the oral administration of MON89034 maize and the occurrence of bladder stones observed in female animals fed the high dose of MON89034.”[6]
ANSES repeated these concerns in a 2017 opinion on another stacked trait maize, MON89034 x 1507 x NK603 x DAS-40278-9.[7]
Risks stack up
As is clear from ANSES’s recent opinions, EU member states’ concerns over MON89034 have not gone away. Rather they have intensified as this GM Bt maize variety has been crossed with other GM Bt maize varieties to produce “stacked” multi-GM-trait crops. The database run by the industry body ISAAA lists a total of 10 GMO maize varieties containing the MON89034 trait that have been approved in Europe for food and feed use.[8]
With stacked trait GM crops, there is a risk that the multiple GMO components of the crop will interact with each other, with other elements in the plant, or with the agrochemicals applied during cultivation, and create new or enhanced toxins or allergens.
However, EFSA does not assess the combined effects of the different GM traits in a stacked variety, nor does it require a 90-day animal toxicity feeding study with the stacked GM crop, such as is required for single-trait GM varieties. Instead EFSA bases its safety assessment on the previous animal feeding studies with the single-trait GM varieties that were crossed to make the stacked-trait variety.[9,10]
Shaky foundation
In 2007 several member states expressed concerns about the safety of a stacked trait GM maize variety containing MON89034, known as MON89034 x MON88017.[11] But EFSA failed to respond to any of these concerns with hard evidence or substantive scientific argument and in 2011 MON89034 x MON88017 was approved for food and feed use.[12]
In a comment that questions whether a solid structure can be built on a shaky foundation, Austria stated that EFSA’s risk assessments of the single traits that went into MON89034 x MON88017 were “insufficient”, so considering an approval for the stacked crop before questions about the single traits had been clarified was not “appropriate”.[11]
Austria also challenged the need for this GM crop by stating that the rootworm pest targeted by the Bt toxins in the GM maize can be controlled by simple crop rotations. It also pointed out that many components of the GM maize were statistically significantly different from the controls and recommended molecular profiling “omics” analysis to detect unintended effects.[11]
France, Austria, and Belgium worried that no animal feeding toxicology studies with the complete stacked trait GM crop had been done, so the safety of the combined Bt toxins had not been tested.[11]
Austria stated, “Despite the new risk of interactions based on gene-stacking no toxicity feeding studies were conducted. The studies carried out on the single events are not regarded as sufficient for the risk assessment of novel constituents and proteins by stacking.”[11]
Belgium stated that a 13-week feeding study in rats should be performed with the stacked trait crop since “synergistic effects” of the combined proteins in the maize “cannot be excluded”.[11]
As GM crops become more complex, skeptical EU member states and the public are likely to grow more mistrustful of EFSA’s failures to address risk and of the Commission’s actions in approving these crops without a democratic mandate.
Claire Robinson is editor of GMWatch.org and the co-author with two genetic engineers of GMO Myths and Truths: A Citizen’s Guide to the Evidence on the Safety and Efficacy of Genetically Modified Crops and Foods, which is available from Amazon.
References
Latham J.R., , Love M., and Hilbeck A. (2017) The distinct properties of natural and GM cry insecticidal proteins. Biotechnology and Genetic Engineering Reviews 33 Pages 62-96.
1. EFSA (2008). Application (Reference EFSA-GMO-NL-2007-37) for the placing on the market of the insect-resistant genetically modified maize MON89034, for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Monsanto[1]. EFSA Journal 909:1-30.
2. EU Commission (2011). COMMISSION DECISION of 17 June 2011 authorising the placing on the market of products containing, consisting of, or produced from genetically modified maize MON 89034 × MON 88017 (MON-89Ø34-3xMON-88Ø1 7-3) pursuant to Regulation (EC) No 1829/2003 of the European Parliament and of the Council.
3. ISAAA (2018). GM crop events approved in European Union.
4. Testbiotech and Genewatch UK (undated). Request for internal review of the market authorisation for genetically engineered maize MON80934 x MON88017, Monsanto, Genuity VT Triple PRO Corn, under Article 10 of Regulation (EC) No. 1367/2006. http://bit.ly/2Eqe9zv
5. EFSA (undated). Application EFSA-GMO-NL-2007-37 (MON89034 Maize). Annex G. Comments and opinions submitted by Member States during the three-month consultation period.
6. ANSES (2016). Saisine no. 2016-SA-0143: Avis de l’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail relatif à une demande d’autorisation de mise sur le marché, au titre du Règlement (CE) no. 1829/2003 relatif aux denrées et aux aliments pour animaux génétiquement modifiés, du maïs génétiquement modifié MIR162 x MON87427 x MON89034 x NK603, développé pour être résistant à certains insectes et tolérant au glyphosate, pour l’importation, la transformation ainsi que l’utilisation en alimentation humaine et animale de cet OGM (dossier no. EFSA-GMO-NL-2016-131). 8 August.
7. ANSES (2017). Saisine no. 2016-SA-0244: Avis de l’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail relatif à une demande d’autorisation de mise sur le marché, au titre du Règlement (CE) no. 1829/2003, du maïs génétiquement modifié MON89034 x 1507 x NK603 x DAS-40278-9, développé pour être résistant à certains insectes et tolérant à plusieurs herbicides (glyphosate, glufosinate-ammonium, 2,4-D et herbicides de la famille des aryloxyphénoxypropionates (AOPP)), pour l’importation, la transformation ainsi que l’utilisation en alimentation humaine et animale de cet OGM (dossier no. EFSA-GMO-NL- 2013-112). 10 February.
8. ISAAA (2018). GM crop events approved in European Union.
9. Then C and Bauer-Panskus (2011). “…ensured that the data were consistent with expectations…”: How industry and EFSA have been systematically undermining the risk assessment of “SmartStax”. Testbiotech.
10. GMWatch (2014). Stacking traits in a GMO is found to cause unexpected synergistic effects. 17 Dec.
11. EFSA (undated). Application EFSA-GMO-NL-2007-39 (Maize MON 89034 x MON 88017): Comments and opinions submitted by Member States during the three-month consultation period.
Adapted from an article that originally appeared in GMWatch.
The photo of bladder stone in a rat on the left is from the US National Toxicology Program; it does not relate to the rat feeding study with MON89034 featured in this article. The photo of an experimental rat on the right is from Understanding Animal Research.
If this article was useful to you please consider sharing it with your networks.



What did FSANZ say about the rat study?
Firstly they said they didn’t require it.
“While FSANZ does not routinely require animal toxicity studies to be undertaken, where
such studies already exist, FSANZ will evaluate them as additional supporting information.”
And in the results they said
“There were no test substance-related deaths or clinical observations during the course of the study. There were no test substance-related effects on body weights and food consumption or haematology, serum chemistry or urinalysis parameters. There were no effects on organ weights attributed to the diets containing MON89034, nor were any test substance-related macroscopic or microscopic findings noted.”
I actually got the full dossier for MON89034. I hadn’t time to look through it when I made the submission though. I can’t find the rat study in it. ??
So, where is the truth? How can the average person know what is safe and not safe when it comes to GMO?
There is no “truth” in science, only evidence, is what I tell people. In this case there is strong doubt about the safety of MON89034. Are you comfortable, in light of that doubt, with feeding it to yourself, your guests, or your children? That is the decision to be made.
I work on probabilities.
If a company works hard to keep their products hidden, unlabelled, such that epidemiological studies become nearly impossible….
If a company knows a large segment of the population doesn’t want to eat their products, yet still puts their unlabelled products in the market place…
If a company hides data, makes it difficult for independent researchers use their materials for independent testing…
If a company doesn’t answer my emails on specific questions related to their product, though I am no competitor…
If a company is required to identify a GM protein in their crop, but can’t find it, and the regulator covers up the fact…
If a company treats their primary clients (farmers) like the scum of the earth (see new GM dicamba crops)…
If a company has a long record of making products that were claimed to be safe but that have been found to harm people…
What’s the probability of any one of their products being likewise harmful?
Madeline, what you said is so true. . . So very true.
You have them pegged 100%.
Let me know if you have updates on this article or fresh findings.